
“Ana Maria Mihalcea, MD, PhD“Ana Maria Mihalcea, MD, PhD
Dec 19, 2023

Image: Figure 2
I am posting this article written by my colleague Dr. Geanina Hagima. She has been attacked in Romania as a conspiracy theorist for researching the presence of nanotechnology in the C19 bioweapon. Yet she has replicated much of my research of different vaccines which show self assembly nanotechnology and has done Electron microscopy and Mass Spectroscopy on Covid 19 vaccines, finding no mRNA. It is my knowingness, that those who tried to attack and discredit us, will soon be known as deceivers of the world. The truth is coming out, and nothing can stop that.
Here is her excellent article:
It is acknowledged in reputable journals that mRNA & Covid vaccines contain nanotechnology: Role of nanotechnology behind the success of mRNA vaccines for COVID-19 and Nanotechnology-based mRNA vaccines, but when a doctor like me says that, it’s considered a conspiracy theory Breaking Fake News.
First it is useful to learn a little about nanotechnology, because although it is widely used in various fields, including medicine, more intensively in the last 20 years, few people, even specialists in their fields, are familiar with the term. Knowledge of this topic is essential to understand what has happened in the last four years, what is being pursued and what solutions we have. The more we ignore this extremely important topic, the fact that this field has long been funded to the tune of billions of euros, that nanotechnology is used in many products despite the lack of regulation and insufficient toxicological assessment, the easier it will be to divert our attention and waste time and energy without finding the causes and effective solutions to the current situation.
What is nanotechnology?
Although there is no unanimously accepted definition, the National Nanotechnology Initiative defines it as the manipulation of matter with at least one dimension sized from 1 to 100 nanometers (nm). Nanotechnology Definition
It is important to know that the properties of materials at the nanoscale differ significantly from those at a larger scale. Nanotechnology is therefore an area that needs to be well defined and regulated.
It is better to use the plural “nanotechnologies” as they include a wide range of technologies and applications – nanomedicine, nanoelectronics, consumer products (e.g. cosmetics, food). In addition to the advantages, nanotechnology raises many issues related to the toxicity and environmental impact of nanomaterials: The European Group on Ethics in Science and New Technologies to the European Commission Opinion on the ethical aspects of nanomedicine (p21).
History of nanotechnology
In 1959 physicist Richard Feynman predicted the possibility of synthesis by direct manipulation of atoms.
The term ‘nanotechnology’ was first used by Norio Taniguchi in 1974.
Nanotechnology became a field in the 1980s with the contribution of K. Eric Drexler.
Fulerenes were discovered in 1985 by Harry Kroto , Richard Smalley and Robert Curl, who together won the Nobel Prize for Chemistry in 1996.
The discovery of carbon nanotubes is attributed to Sumio Iijima in 1991.
In the early 2000s, the field attracted scientific, political and commercial attention. Controversy has arisen over the definitions and potential implications of nanotechnologies. Governments began to promote and fund nanotechnology research.
Funding
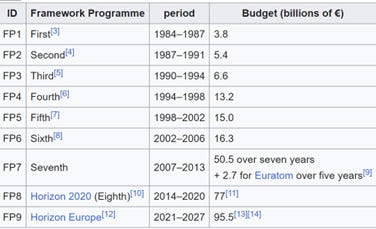
In the US, the National Nanotechnology Initiative created a size-based definition of nanotechnology and established funding for nanoscale research. The European equivalent of the US initiative is the European Framework Programmes for Research and Technological Development, which have funding in the billions of euros (Figure 1).
Of the funds allocated to these Framework Programmes for Research and Technological Development, nanotechnology has been allocated more than €1.36 billion (550 projects funded) through FP6 (2002-2006), about €3.5 billion through FP7 (2007 – 2013) and about €2 billion through FP8 (2014-2020). For FP9 (2021- 2027) I could not find any data on nanotechnology funding, which certainly received a few billion euros more than in previous years. The countries involved in FP9 are, in addition to EU Member States, non-EU countries such as: Albania, Armenia, Bosnia and Herzegovina, Faroe Islands, Georgia, Iceland, Israel, Kosovo, Moldova, Montenegro, New Zealand, Macedonia, Norway, Serbia, Tunisia, Turkey, Ukraine, UK .
Figure 1. The Framework Programmes for Research and Technological Development , also called Framework Programmes or abbreviated FP1 to FP9, are funding programmes created by the European Union / European Commission to support and encourage research in the European Research Area (ERA). Since 2014, the funding programmes have been called Horizon
It is important to bear in mind that nanotechnology is the first of the objectives of the World Economic Forum’s Fourth Industrial Revolution, alongside brain research, 3D printing, mobile networks, computerisation (Figure 2)
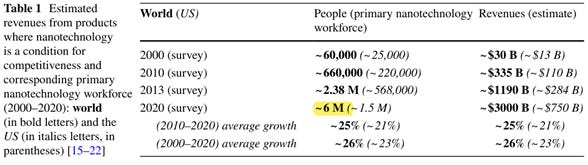
In addition to the enormous funding, the income of workers in this field averages $500,000 annually, and the number of workers has grown from 60,000 in 2000 to 6 million in 2020 (Figure 3)
Figure 3
Nanomaterials – special properties
At the nanoscale the properties of particles change, quantum and surface phenomena of matter occur. The quantum size effect consists in modifying the electronic properties of solids at nanometric dimensions.
It also changes a number of physical properties – mechanical, electrical, optical, etc. – compared to macroscopic systems, allowing unique applications. At nanoscale: copper, an opaque material, can become transparent; aluminium, a macroscopically stable material, can become flammable; gold, an insoluble material, can become soluble and a powerful chemical catalyst .
The evolution of nanotechnology
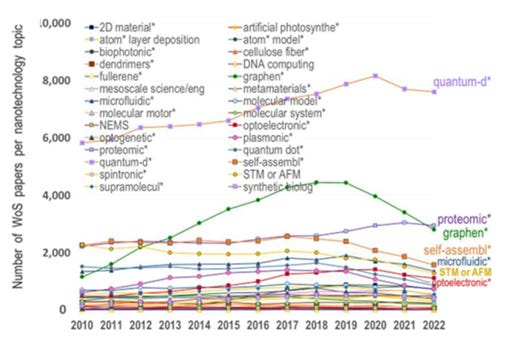
Nanotechnology evolved from passive nanostructures in 2000, to active nanostructures after 2005, to nanosystems after 2010, molecular nanosystems after 2015 and converging technologies after 2020, as described by Romanian-born engineer Mihail Roco , one of the principal architects of the US National Nanotechnology Initiative (Figure 4) , In 2020-2022, significant progress has been made in the areas of quantum, graphene, proteomics, micro/nanofluidics and optoelectronics.
Perhaps it is useful to know some facts about Mr. Mihail Roco as well. He has played and continues to play an important role in the field of nanotechnology worldwide, and was the principal author of the strategy for the first US national nanotechnology programme, launched in 2000 by President Bill Clinton. Professor Roco was also involved in the initiation and development of nanotechnology research in Romania. Since 2011, Mihail Roco is Doctor Honoris Causa of the Polytechnic University of Bucharest, which he graduated in 1970, and since 2012 he is an honorary member of the Romanian Academy, Section of Science and Information Technology .
As far back as 2006 , an expert group of the European Medicines Evaluation Agency (EMEA) notes significant progress in nanotechnology: “Novel applications of nanotechnology include nanostructure scaffolds for tissue replacement, nanostructures that allow transport across biological barriers, remote control of nanoprobes, integrated implantable sensory nanoelectronic systems and multifunctional chemical structures for drug delivery and targeting of disease.” (page 14)
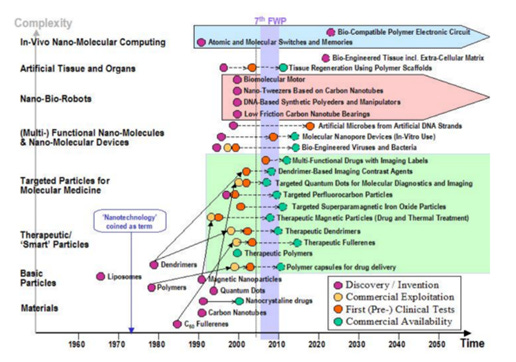
In 2010 a variety of nanoparticles were commercially available such as dendrimers, quantum dots, superparamagnetic iron oxide particles, magnetic particles, fullerenes, polymers (Figure 5) – page 15.
Figure 4. Evolution of nanotechnology (page 15)
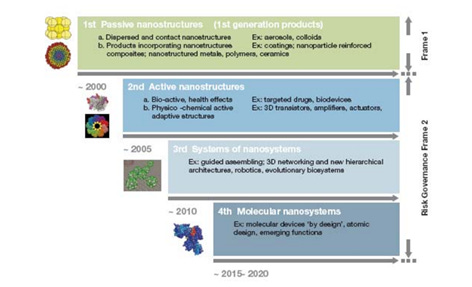
Figure 5. Evolution of nanotechnology (page 15)
The number of publications in the field of nanotechnology has been steadily increasing over the last 10 years, with most articles on quantum dots, graphene, proteomics, self-assembly (Figure 6) .
Figure 6. Publications on nanotechnology
Nanotechnology – toxicological aspects
In addition to its advantages, nanotechnology also has disadvantages in terms of toxic effects, which are important to monitor in view of its use for diagnostic, therapeutic or cosmetic purposes.
Adverse effects may be due to :
– accumulation in tissues or organs – liver, cardiovascular, renal and nervous system
– impaired metabolism
– conformational changes in proteins with possible prion diseases
– stimulation of tumour development through DNA damage
In addition, ” known and widely accepted toxicological methods are not sufficient to detect possible damaging effects of nanoparticles.” (Page 21).
In vivo use of NPs may result in inflammatory responses, cellular toxicity, unexpected distribution and elimination, and insufficient transport to a specific target. These unfavorable phenomena may largely be related to the in vivo protein-NP interaction, termed “protein corona.”. “The ‘corona effect’ affects the biological behavior of NPs and changes their functionality, occasionally resulting in loss-of-function or gain-of-function” .
The legal situation – Nanotechnology is not regulated for 20 years
In 2007 there was still no specific legislation on nanomedicine in EU Member States. It was acknowledged that ” The lack of a clear legal definition of nanomedicine, and the fact that current regulation is based on other characteristics where nanomedicine was not part of the considerations on which the wording was based, present a problem, as it may be unclear whether nanomedicine is to be placed within or outside the scope of certain legislation. Some nanomedicinal innovations may fall within several categories of regulation which may apply simultaneously. For example, nanomedical products may combine different mechanisms of action, be they mechanical, chemical, pharmacological or immunological. There may therefore be a risk not only of uncertainty as to which regulation(s) are applicable, but also of there being a plethora of regulatory provisions that are of relevance. Both situations are problematic from a legal point of view.” (p 23, 33)
The lack of regulation of nanotechnology persisted in 2020 despite repeated calls from the scientific community. Regulation is essential to provide legal certainty to manufacturers, policy makers, healthcare providers and the general public. There is a lack of an internationally accepted definition of nanomaterials. The National Institutes of Health, USA, the European Science Foundation and the European Technology Platform have different definitions and the FDA has no clear definition.
An EAASM scientific report (Sept 2020) calls for the development of a scientific consensus on definitions for nanomedicines in Europe. The report calls for the adoption of a centralised procedure by the EMA for all nanomedicines and nanosimilars to better review these products .
In 2021 Peter Vitanov, member of the European Parliament, claimed that innovative mRNA vaccines contain nanoparticles. He believes that ” Assembling different chemical parts into complex nanoparticles requires highly standardised and complex manufacturing processes that can guarantee consistent quality, clinical effectiveness and safety […] Changes in quality, size distribution, surface properties, drug loading and release profiles, aggregation status and stability can alter how a nanomedicine acts within the body with a significant impact on patient safety and efficacy.”
An EAASM scientific report made key recommendations to ensure patient safety and enable the EU to fully exploit the potential of nanotechnology . The report called for the development of a scientific consensus on nanomedicine definitions in Europe, improving education and promoting awareness of the complexity and sophistication of nanomedicine among policy makers, prescribers, payers and patients. It also called for the adoption of a centralised procedure by the European Medicines Agency (EMA) for all nanomedicines and nanosimilars, which would ensure better scrutiny of these complex products.
In November 2022, the online information poster for a debate on the regulation of nanomedicine in the EU stated “The regulatory framework for nanomedicines presents critical issues that have not been fully resolved, from adopting a definition of nanomedicine that is harmonised at EU level, to developing protocols and common guidelines for the characterisation, evaluation and control of the nanomedicine production process.”
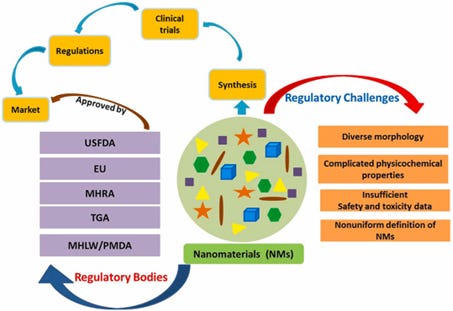
In a February 2023 article on the regulation of nanomaterials and nanomedicine it is revealed that nanotechnology is increasingly being used in almost all scientific fields, especially in the pharmaceutical field, and that it is of vital importance . At the same time it is stated that there are concerns among the public and scientific communities about their quality, safety, efficacy and toxicity, but “due to the complex nature of nanomaterials, the development of legislation and standards in this area is a particular challenge for regulators” (figure 7). It is also noted that most nanomaterials function by interacting at the biomolecular level with cellular components and genetic materials, which directly and indirectly influences genomic function, and that this could have both positive beneficial therapeutic effects and negative effects such as genotoxicity and genetic mutations, which could prove lethal and fatal to humans. At the same time, it is noted that most of the norms and regulations currently in force relate to materials of common size.
Figure 7. Regulatory issues of nanomaterials
Nanotechnology in covid „vaccines”. Personal investigations reveal differences in composition from that declared by producers
As I mentioned at the beginning of the article, it is recognised that experimental mRNA ‘vaccines’ contain nanotechnology, which, according to the studies presented, can cause various adverse effects including DNA damage. However, the package leaflet of these experimental products clearly states that no carcinogenicity and genotoxicity studies have been carried out because it was assumed that these products have no genotoxic potential (page 18). Although the manufacturers of these products knew about these regulatory issues regarding nanotechnology, they were nevertheless approved, distributed and presented as “safe and effective”. Therefore, I believe that this important observation may be useful in legal actions against both the manufacturers and those who presented them to the public as safe.
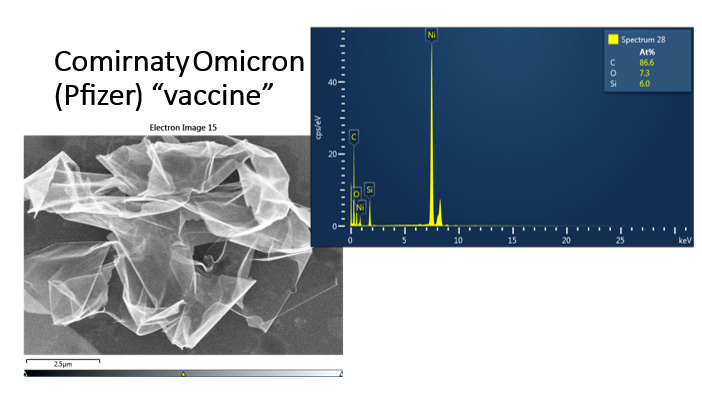
In addition to these ‘oversights’ by manufacturers, I have identified another problem. With the help of specialists, I carried out an energy-dispersive X-ray spectroscopy analysis of two mRNA products Comirnaty Omicron and Moderna (figure 8,9,10,11). We found that both products contained mainly carbon, oxygen and silicon atoms, without identifying nitrogen and phosphorus atoms, as would be normal if these products contained mRNA or DNA. In addition, in the Comirnaty Omicron product we also identified magnesium, titanium, and a rare element, yttrium, which is used in nanoelectronics , optoelectronics , yttrium silicate being used to produce anticovid disinfectants that are activated by natural light or LED light . In the Moderna product we also identified atoms of titanium, tin, aluminium, magnesium. I think it is not by chance that the FP9 program (2021-2027) invests a lot in the field of photonics . Silicon is known to be used to produce nanosensors, biocompatible quantum dots , . In addition, slicon, yttrium, titanium, aluminium, tin, magnesium are not reported in the leaflet of these products. Therefore, these products contain elements other than those declared in the leaflet, probably only nanotechnology. As for the absence of nitrogen and phosphorus (i.e. mRNA or DNA) one can argue that there are batch variations, but what was the likelihood of encountering two different products and none of them containing these atoms?
Given this data , and that of other researchers , Steve Kirsch Pfizer Analysis and German Working Group, I have reason to believe that these products only contain nanotechnology that is intended to be used for the internet of bodies network and the brain computer connection, given that nanoparticles cross the blood brain barrier. In addition to anticovid products, I also analysed a dental anaesthetic (Artidental) (figure 12, 13) and a classical vaccine (Prevenar pneumococcal vaccine) (figure 14, 15) in which we identified the presence of a high percentage of silicon atoms (which do not appear in the prospectus). It is therefore very likely that other injectable products also contain nanotechnology that can be used for monitoring purposes. That is why I think it is extremely important that other researchers also do such investigations, to bring the truth to light, to identify what we are dealing with, so that we can find solutions.
For a better understanding of how medicine worked during covid I think it’s helpful to mention the surprisingly honest but cynical statement by a former Pfizer vaccine research and development director, Kathrin Jansen, “We flew the aeroplane while we were still building it” (figure 16) .

The article continues, but I am unable to post it due to substack limitations.
Here is the full PDF:
We Fly The Plane While We Build It Toxicology And Regulation In Nanomedicine
3.52MB ∙ PDF file
Humanity United Now – Ana Maria Mihalcea, MD, PhD is a reader-supported publication. To receive new posts and support my work, consider becoming a free or paid subscriber.”